I. Eye Preparation |
||
| Icool | FDA Approved No. DR-XY42792 Olopatadine 0.1% 5ml Eye Drops
Aju Pharm Co., Ltd. – Korea |

|
| Iflox | FDA Approved No. DR-XY44308 Levofloxacin Eye Drops, 5ml (Preservative Free)
Unimed Pharmaceuticals, Inc. – Korea |

|
| Ivisc | FDA Approved No. DR-XY36029 Sodium Hyaluronate Eye Drops 0.1%, 5ml
Unimed Pharmaceuticals, Inc. – Korea |

|
| Iradex | FDA Approved No. DR-XY38880 Tobramycin + Dexamethasone, 5ml 3mg + 1mg/ml drops |
|
| Ipodex | FDA Approved No. DRP-442-03 Polymixin + Neomycin + Dexamethasone, 5ml 6,000 + 5mg + 1mg/ml, drops |
|
| Itearz | FDA Approved No. DRP-498-03 Hypromellose 0.3%, 10ml 3mg/ml, drops |
|
| Iclenz | FDA Approved No. DRP-1226-01 Potassium Iodide + Sodium Iodide, 10ml 3mg + 3mg/ml, drops |
|
| Ipredniz | FDA Approved No. DRP-804-02 Prednisolone Acetate 1%, 5ml 10mg/ml, drops |
II. Eye Vitamins |
||
| I-Vites | FDA Approved No. FR-4000001040285 Lutein 6mg + Zeaxanthin 6mg + Zinc 4.5mg + Selenium Tablet 31mcg + Ascorbic Acid 75mg + Vitamin E 10iu + Vitamin A 30iu |

|
| I-Vites MAX | FDA Approved No. FR-4000003805615 Lutein 33mg + Bilberry 60mg + Marigold 30mg + Beta-Carotene 6mg + Rhodiola Capsule 5mg
Lita Pharmacy Co., LTD. – Taiwan |

|
III. Trifocal Intra-Ocular Lens |
| Triva | FDA Approved No. MDR-10968B Trifocal Diffractive Aspheric Yellow Hydrophilic Acrylic Length: 12.5mm Optic: 6.0mm A Constant(Optical): 118.4 ; (Acoustical): 118.1 Add: 1.75/3.5
HumanOptics – Germany |
 |
IV. Multifocal Toric Intra-Ocular Lens |
| ToricaDiff | FDA Approved No. MDR-07384B Multifocal Toric Length: 12.5mm Optic: 6.0mm A Constant(Optical): 118.4 ; (Acoustical): 118.1 Add: 3.5 SE 21.75
HumanOptics – Germany |
V. Multifocal Intra-Ocular Lenses |
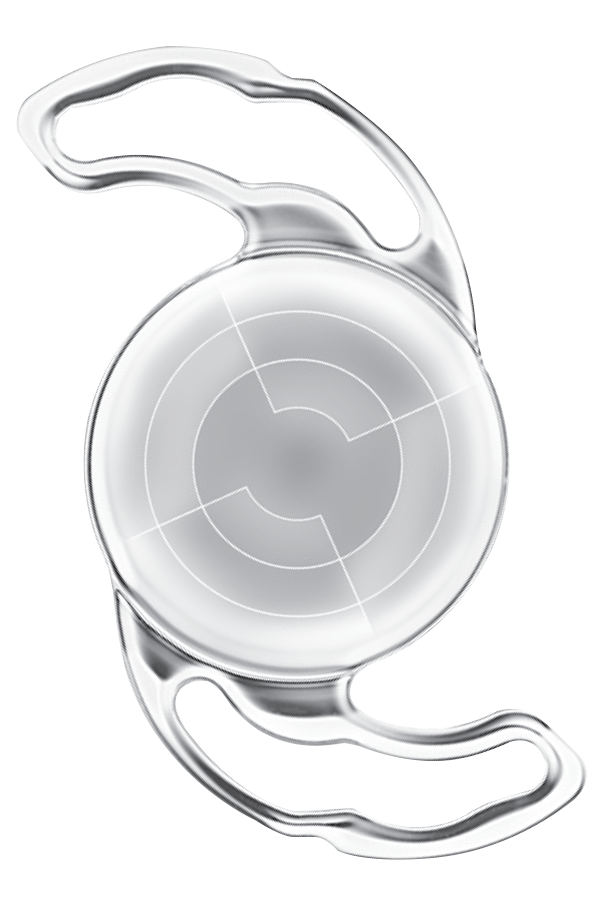
| Precizon NVA | FDA Registration No. MDR-10034B Multifocal Refractive Aspheric Presbyopic Length: 12.5mm Optic: 6.0mm A Constant(Optical): 118.8 Add: 2.75D
Ophtec BV – The Netherlands |
 |
VI. Toric Intra-Ocular Lens |
| Torica | FDA Approved No. MDR-07384A Monofocal Toric Aspheric Hydrophilic Acrylic Yellow Length: 12.5mm Optic: 6.0mm A Constant(Optical): 118.4 ; (Acoustical): 118.1
HumanOptics – Germany |
VII. Monofocal Intra-Ocular Lenses |
| Aspira | FDA Approved No. DVR-7952 Aspheric Yellow Hydrophilic Acrylic Length: 12.5mm Optic: 6.0mm A Constant(Optical): 118.4
HumanOptics – Germany |
 |
| Aspira-aXA | FDA Approved No. MDR-09050A Aspheric Clear Hydrophilic Acrylic Monofocal Length: 11.0mm Optic: 7.0mm A Constant(Optical): 118.3
HumanOptics – Germany |
 |
| Precizon Monofocal | FDA Approved No. MDR-10215 Aspheric Clear Monofocal Length: 12.5mm Optic: 6.0mm A Constant(Optical): 118.6
Ophtec BV – The Netherlands |
 |
| Neo Eye RAF-22L | FDA Approved No. MDR-06131 Hydrophilic Aspheric Acrylic Length: 12.5mm Optic: 6.0mm A Constant(Optical): 118.0
P.T. Rohto – Japan / Indonesia |

|
| Neo Eye RF-22L | FDA Approved No. DVR-5134 Hydrophilic Spheric Acrylic Length: 12.5mm Optic: 6.0mm A Constant(Optical): 118.0
P.T. Rohto – Japan / Indonesia |

|
| Ocuflex ANU6 | FDA Approved No. MDR-09349B Spheric Clear Hydrophilic Acrylic Length: 12.5mm Optic: 6.0mm A Constant(Optical): 118.0
CareGroup – India |
|
| Asphire Gold HD | FDA Approved No. MDR-09374A Monofocal Aspheric Yellow Hydrophilic Acrylic Length: 12.0mm Optic: 6.0mm A Constant: 118.0
Excel Optics- India |
|
| Trufold | FDA Approved No. MDR-09374B Monofocal Spheric Hydrophilic Acrylic Length: 12.0mm Optic: 6.0mm A Constant: 118.0
Excel Optics- India |
VIII. PMMA Intra-Ocular Lenses |
| Neo Eye RP-11 | FDA Approved No. DVR-3300 Posterior Chamber Length: 12.0mm Optic: 5.25mm A Constant: 118.1
P.T. Rohto – Japan / Indonesia |
| Neo Eye RE-03PH | FDA Approved No. DVR-3300 Posterior Chamber Length: 13.0mm Optic: 6.5mm A Constant: 118.2
P.T. Rohto – Japan / Indonesia |
IX. Special Intra-Ocular Lenses |
| Artisan Aphakia 5/8.5 | FDA Approved No. MDR-10112 Iris Fixated; Plan-Convex Length: 8.5mm Optic: 5.4mm A Constant Optical SRK-T: Anterior Implantation: 115.7 Posterior Implantation: 116.9
Ophtec BV – The Netherlands / Indonesia |
X. Viscoelastics |
|
| Pe-ha-luron | FDA Registration No. MDR-08787B Sodium Hyaluronate 3.0%, 1ml
FDA Registration No. MDR-09652 FDA Registration No. MDR-08787A Albomed GmbH – Schwarzenbruck, Germany |
| Curamed | FDA Registration No. MDR-04696 Sodium Hyaluronate 1.8%, 1.5ml
Curamed Ophthalmics – The Netherlands |
| Curagel | FDA Registration No. DVR-8812 HydroxyPropylMethylCellulose Solution 2%, 2ml
Curamed Ophthalmics – The Netherlands |
| Prolonn | FDA Registration No. MDR-10449 Sodium Hyaluronate 1.8%, 1ml
Ophtechnics Unlimited – Haryana, India |
| Hiluron | FDA Registration No. MDR-10601B Sodium Hyaluronate 1.8% 1ml
CareGroup – India |
| ProGell | FDA Registration No. MDR-10448 HydroxyPropylMethylCellulose Solution 2%, 2ml
Ophtechnics Unlimited – Haryana, India |
XI. Knives and Sutures |
| Mani Knife | FDA Approved No. MDR-05086A 15 Degree Stab Knife
FDA Approved No. MDR-05086B FDA Approved No. MDR-05086D FDA Approved No. MDR-05086C FDA Approved No. MDR-05086E FDA Approved No. MDR-05086F FDA Approved No. MDR-05086G Mani – Japan |
| Mani Sutures | FDA Approved No. MDR-06200 10-0 Needled Sutures Nylon
FDA Approved No. MDR-08010 FDA Approved No. MDR-06199 Mani – Japan |
XII. Other Products |
AmnioTek™ |
|
| AmnioTek™2 | FDA Registration No. MDR-09052A AmnioTek™2 is a double layered graft so it can be put in any orientation as both surfaces are stromal surfaces. The size of each piece of AmnioTek™2 is 3 x 3 cm.
ISP Surgical, USA |
| AmnioTek™-C | FDA Registration No. MDR-09052B AmnioTek™-C is a proven effective treatment for a host of ocular surface conditions. Each disc of AmnioTek™-C is 12 mm in diameter.
ISP Surgical, USA |
Eye Drapes |
|
| Pre-Cut | FDA Registration No. MDR-06872B Bioflex Eye Drape with Pouch 100cm x 120cm (EDP100120PC)
Surgitech Innovation, India |
| Non Pre-Cut | FDA Registration No. MDR-06872A Bioflex Eye Drape with Pouch 100cm x 120cm (EDP100120)
Surgitech Innovation, India |
Trypan Blue |
|
| Blue Rhexis | FDA Registration No. MDR-09446A Trypan Blue Solution 0.06% Sterile Solution (1mL vial)
Contacare Ophthalmics and Diagnostics, India |
| IO-Blue | FDA Registration No. MDR-06707 IO-BLUE Trypan Blue Ophthalmic Solution (1mL vial)
Surgitech Innovation, India |
